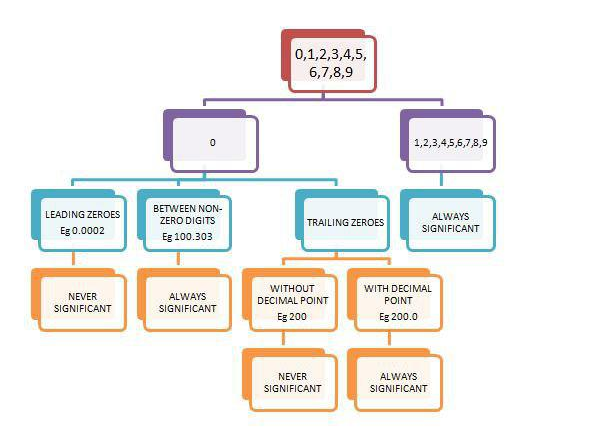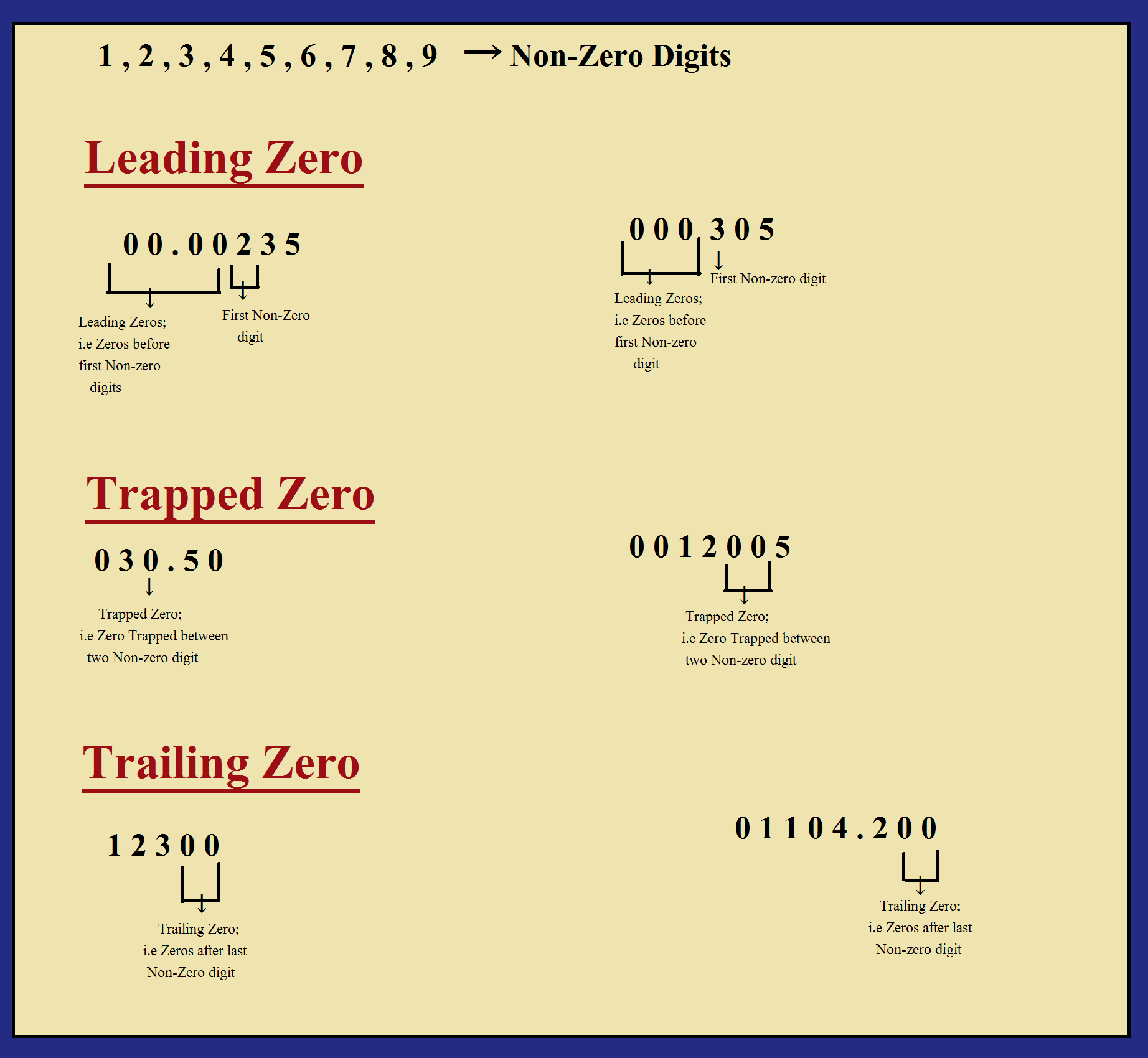
Defination : (1). A leading zero is any 0 digit that comes before the first nonzero digit
(2). trailing zeros are a sequence of 0's after which no other digit follows.
(i) All non-zero digits are significant.
(ii) Zeros preceding to first non-zero digit are not significant. These zeros only indicates the position of decimal point only.
(iii) Zeros between two non-zero digit are significant.
(iv) Zero at the end or right of a number are significant if they are on the right side of the decimal point.
e.g. `0.200g` has `3` significant figures.
If there is no decimal point then the terminal zeros are not significant.
e.g. `tt((text{Number} , text{no. of significant figures} ) , (100 , 1) , (100. , 3) , (100.0 , 4))`
(v) Objects which can be counted have infinite significant figure as these are exact numbers. And can be represented as writing infinite number of zero after placing a decimal.
e.g. `2 = 2.000000` or `20 = 20.00000`

Defination : (1). A leading zero is any 0 digit that comes before the first nonzero digit
(2). trailing zeros are a sequence of 0's after which no other digit follows.
(i) All non-zero digits are significant.
(ii) Zeros preceding to first non-zero digit are not significant. These zeros only indicates the position of decimal point only.
(iii) Zeros between two non-zero digit are significant.
(iv) Zero at the end or right of a number are significant if they are on the right side of the decimal point.
e.g. `0.200g` has `3` significant figures.
If there is no decimal point then the terminal zeros are not significant.
e.g. `tt((text{Number} , text{no. of significant figures} ) , (100 , 1) , (100. , 3) , (100.0 , 4))`
(v) Objects which can be counted have infinite significant figure as these are exact numbers. And can be represented as writing infinite number of zero after placing a decimal.
e.g. `2 = 2.000000` or `20 = 20.00000`